About Us
We are a dedicated team of biomedical scientists and medical doctors with extensive experience in establishing biobanking projects and customized pre-clinical biospecimen collection studies.
We offer expertise in:
- Biobanking
- Regulatory Affairs
- Quality Assurance and Control
- Clinical and Laboratory Research
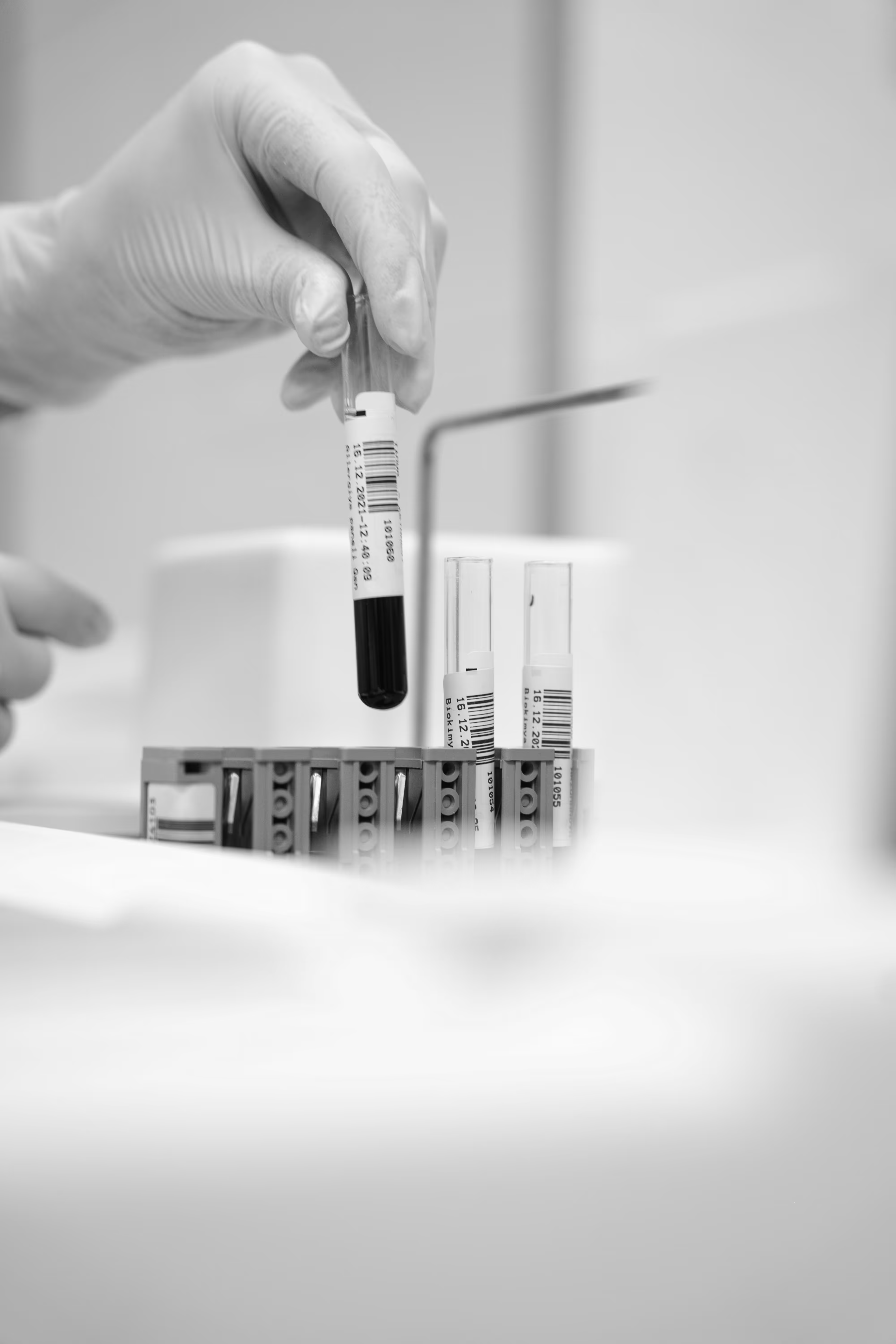
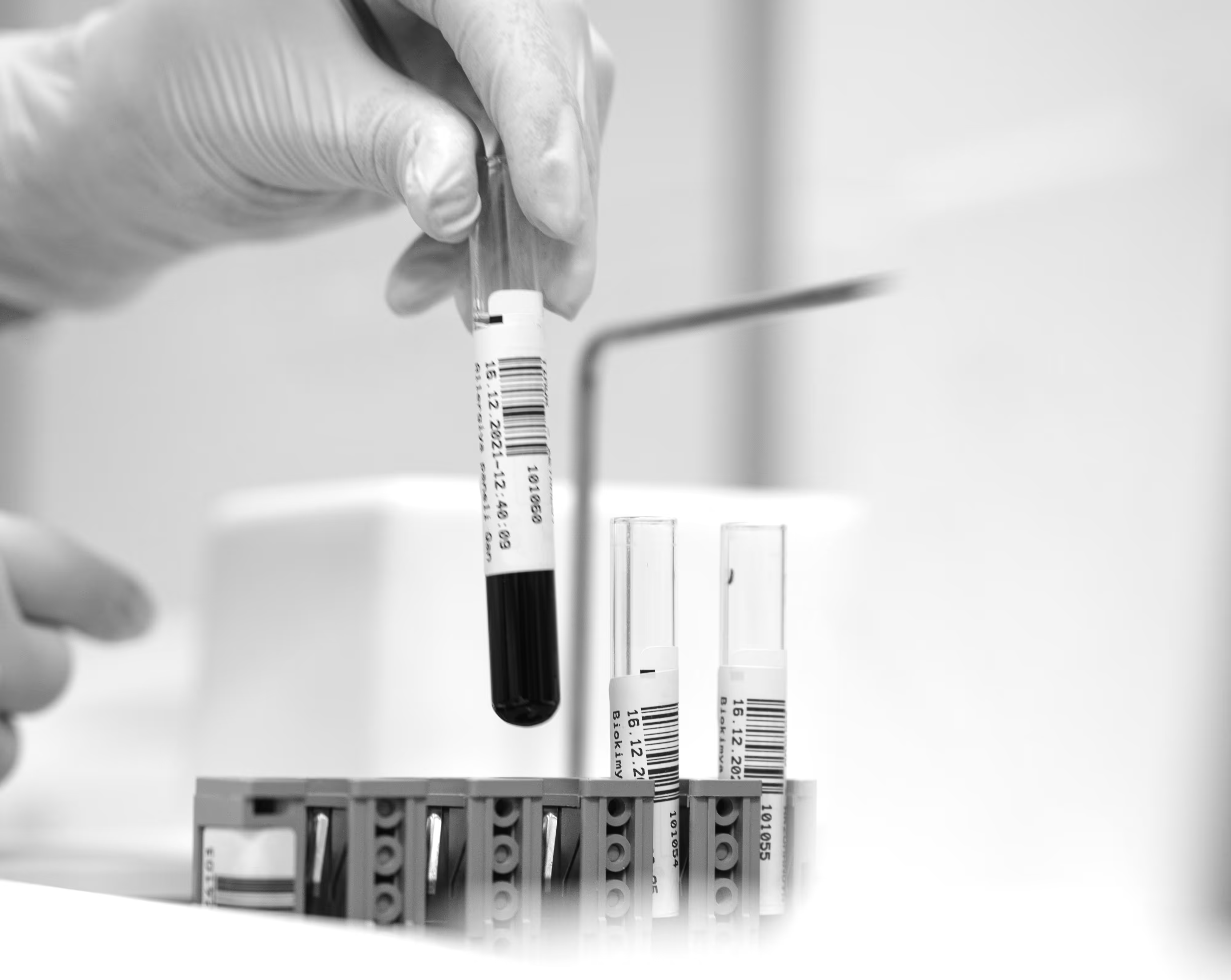
Your research is in the capable hands of our experienced professionals.
- David Shahbazian, DIRECTOR
Our transparent business model effectively connects pharmaceutical clients to the biobanking partners. This approach fosters direct collaboration between scientists on technical, medical, and scientific aspects of patient cohort selection and biospecimen collection, ensuring that your project specifications are thoroughly understood and precisely followed.

Dr. David Shahbazian has been leading ART-Biopharma as Executive Director since its inception in 2020, specializing in Business Development, Customer Relations, Multicenter Project Management, and strategic international partnerships.
With nearly a decade of experience, David previously served as Project Director at Trans-Hit Biomarkers Inc., where he was responsible for establishing relationships with new academic and commercial biobanks. David has a robust scientific background in molecular oncology, which he developed during his postdoctoral training in the Departments of Pharmacology and Medical Oncology at Yale University (New Haven, CT, USA) and at Georgetown University Lombardi Cancer Center (Washington, DC, USA). His research focused on breast cancer, melanoma, and pancreatic ductal adenocarcinoma, particularly in the context of signal transduction and immuno-oncology.
David's academic work has resulted in the publication of over 20 peer-reviewed scientific research articles, invited reviews, and book chapters. He holds a PhD in Biochemistry from McGill University (Montreal, Quebec, Canada).
David is dedicated to assisting ART-Biopharma's customers with their most challenging requests. His motto is, "We will make every possible effort to advance your experimentation and science, since progress cannot wait!"

Dr. Eva Simone Jordan joined ART-Biopharma in May 2024 as a Project Director, specializing in Account Management, Project Management, and Strategic Partnerships.
Eva brings nearly a decade of experience, having served as a Senior Project Manager at Trans-Hit Biomarkers Inc. This role was followed by positions as Strategic Biospecimen Sourcing Manager and Team Lead Manager at Azenta Life Sciences Sample Sourcing, where she led a team of Scientific Study Managers. Eva has a robust background in biobanking, gained from her time at the Nottingham Health Science Biobank, where she progressed from Senior Biobank Scientist to Biobank Business Manager.
She holds a PhD in Biochemistry and Molecular Biology from the University of Leicester (United Kingdom). Eva is adept at fostering client relationships, driving business growth, and enhancing strategic planning and operational efficiency.

Dr. Titan Asatryan graduated from Yerevan State Medical University in 2018 and subsequently served as a Military Doctor in the Armed Forces of the Republic of Armenia. From 2018 to 2021, he worked as a General Practitioner (GP) with the Brigade Support Medical Company, where he held several key positions. During this time, Dr. Asatryan went on to become a decorated war veteran and completed the Captain's Career Course at the U.S. Army Medical Center of Excellence in Texas, USA. Following this achievement, he was promoted to the role of Brigade Surgeon.
In 2022, Dr. Asatryan joined Art-Biopharma LLC as a Project Manager. His exceptional professional and communication skills were instrumental in establishing ART-Biopharma’s network of partnered clinical sites in Armenia. He has managed complex biobanking studies, coordinating efforts among multiple teams of clinical investigators. Currently, Dr. Asatryan serves as the Project Director at ART-Biopharma, based in London, UK.
As of 2024, Dr. Asatryan is also completing hispostgraduate studies towards a Master’s in Public Health (MPH) degree at King'sCollege London, UK.

Dr. Hasmik Nersoyan earned her Medical Doctor degree from Yerevan State Medical University in 2019. She later pursued postgraduate studies and obtained a Master's in Public Health (MPH) from the same institution. In 2021, she completed her residency program, specializing in gastroenterology. Dr. Nersoyan has garnered valuable experience at the Mikaelyan Institute of Surgery and the National Center of Oncology of Armenia, further honing her skills and deepening her passion for healthcare.
Dr. Nersoyan joined Art-Biopharma as a Project Manager in 2023. In this role, she combines her medical expertise with project management skills, demonstrating a strong commitment to driving impactful international initiatives. She plays a crucial role in advancing numerous biomedical studies commissioned by ART-Biopharma’s pharmaceutical partners.

Dr. Vahagn Beybutyan has been a Project Manager at ART-Biopharma since 2023. He earned his Doctor of Medicine degree from Yerevan State Medical University in 2020 and specialized as a vascular surgeon in 2023. His medical practice includes positions at the Ministry of Health of Armenia, the National Center for Burns and Dermatology, and the Mikaelyan Institute of Surgery in Yerevan, Armenia.
Dr. Beybutyan’s background in both clinical practice and healthcare management enables him to effectively bridge the gap between medical practice and project management. At ART-Biopharma, he leverages his professional skills to oversee and advance various strategic initiatives, ensuring the successful execution of pre-clinical projects. His contributions are instrumental in fine-tuning ART-Biopharma studies that require coordination between multiple stakeholders, including ethics committees, administration, sample collection/processing, and logistics.


